In our continuous journey towards understanding and combating complex diseases, Monoclonal Antibody Therapy has emerged as one of the most significant innovations reshaping the landscape of modern medicine.
For many years, treatments often relied on a general approach, broadly targeting cells, which could affect healthy tissues alongside diseased ones.
However, monoclonal antibody therapy introduced a new concept: extreme precision.
It is akin to designing unique keys that fit specific locks on diseased cells, allowing for targeted and effective treatment while minimizing collateral damage.
These laboratory-designed antibodies are not just drugs; they are intelligent biological tools, capable of recognizing specific molecules with exceptional accuracy.
Monoclonal antibody therapy represents a beacon of hope for millions worldwide, opening vast avenues for treating previously intractable diseases.
It represents a true revolution in precision medicine.
With its continuous development and application in Liva Hospital and other global institutions, it has become a turning point in the treatment of cancer, autoimmune diseases, and even viruses.
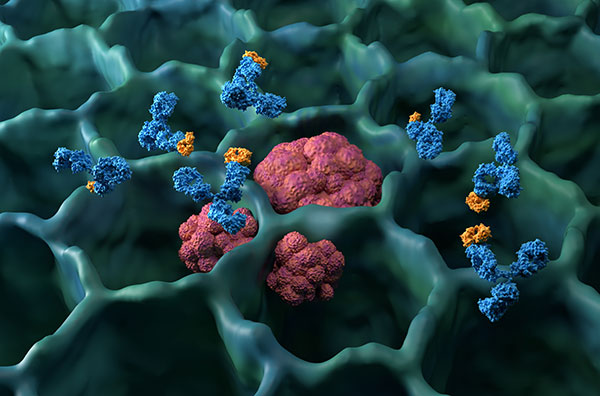
Understanding Antibodies
To understand monoclonal antibody therapy, we must first grasp the vital role of natural antibodies in our bodies.
Antibodies are specialized proteins produced by the immune system in response to the presence of foreign and harmful substances, known as antigens.
These antigens can be proteins on the surface of bacteria and viruses, cancer cells, or even allergens.
Each antibody is uniquely designed to bind to one specific antigen, just like a lock and key.
Upon binding to the antigen, antibodies directly neutralize the harmful substance, mark it for easier destruction by other immune cells, or stimulate a broader immune response.
In the human body, B-cells produce countless diverse antibodies, each targeting a different antigen.
But in monoclonal antibody therapy, scientists use advanced techniques to produce large quantities of only one type of antibody, precisely targeting a specific antigen.
This allows the treatment to focus on the diseased target without broadly affecting healthy cells, mimicking the precision of the natural immune system but with unprecedented guidance and control.
Manufacturing Monoclonal Antibodies
The manufacturing of monoclonal antibodies is an astonishing achievement in biological engineering, relying on complex techniques to produce large quantities of a single, genetically identical antibody.
The process, developed by Georges Köhler and César Milstein in the 1970s, for which they received a Nobel Prize, begins by immunizing an animal (usually a mouse) with the specific antigen the scientists wish to target.
The mouse’s immune system responds by producing B-cells that generate antibodies against this antigen. These B-cells are then isolated from the mouse’s spleen.
The challenge is that B-cells cannot grow and divide in the laboratory for long periods. To overcome this, these B-cells are fused with cancer cells known as “myeloma cells,” which have the ability to proliferate indefinitely.
This fusion results in hybrid cells called “hybridomas.” These hybridomas possess the characteristics of both parent cells: they produce the desired antibody (from the B-cell) and have the ability to divide and multiply indefinitely in a laboratory environment (from the myeloma cell).
Next, the hybridomas are screened to select the single clone that produces the desired antibody with the highest quality and quantity.
This selected clone is then grown in large cell cultures to produce vast quantities of monoclonal antibodies, which are purified and modified to be suitable for human use, reducing immune reactions against them.
Mechanisms of Action of Monoclonal Antibody Therapy
Monoclonal antibodies do not work through a single mechanism; instead, they can use multiple, diverse ways to combat diseases, depending on the antigen they target. These mechanisms make them flexible and powerful therapeutic tools:
- Direct Blocking and Inhibition: Some monoclonal antibodies bind to specific receptors on the surface of diseased cells, such as growth receptors.
Upon binding, these antibodies prevent the antigen from sending growth signals, leading to the cessation of diseased cell proliferation or inducing their death. An example is Trastuzumab (Herceptin), which blocks the HER2 receptor in some types of breast cancer. - Immune System Activation: Monoclonal antibodies can act as a “tag” for diseased cells, making it easier for other immune cells in the body (such as natural killer cells and macrophages) to recognize and destroy them.
This includes mechanisms like Antibody-Dependent Cellular Cytotoxicity (ADCC) and Antibody-Dependent Cell Phagocytosis (ADCP). - Drug Delivery: Some monoclonal antibodies are used as “guided missiles” carrying toxic substances (such as chemotherapy or radiotoxins) directly to the diseased cells that bear the targeted antigen.
This reduces the exposure of healthy cells to the toxic drug and minimizes systemic side effects. These treatments are known as “Antibody-Drug Conjugates (ADCs).” - Immune Checkpoint Modulation: Certain monoclonal antibodies target immune checkpoints (such as PD-1 or CTLA-4) to release the immune system from suppressing cancer cells. This is the basis of modern immunotherapy that enhances the body’s ability to attack cancer.
- Neutralizing Pathogens: In infectious diseases, antibodies can directly bind to viruses or bacteria, preventing them from infecting cells or neutralizing their toxins.
Applications of Monoclonal Antibody Therapy
Monoclonal antibody therapy has revolutionized the treatment of a wide range of diseases and opened up previously unforeseen possibilities.
The most prominent of these applications are in the field of cancer treatment. Monoclonal antibodies are used to target specific proteins on the surface of cancer cells, such as HER2 in breast cancer, or EGFR in colon and lung cancer, or CD20 in lymphoma.
These treatments have led to significant improvements in survival rates and quality of life for many cancer patients.
In addition to cancer, monoclonal antibodies play a crucial role in treating autoimmune and inflammatory diseases. In these diseases, the immune system mistakenly attacks healthy body tissues.
Antibodies target specific proteins or pathways that contribute to inflammation and tissue damage. Examples include the treatment of:
- Rheumatoid Arthritis: Antibodies like Infliximab or Adalimumab target Tumor Necrosis Factor alpha (TNF-alpha).
- Psoriasis and Psoriatic Arthritis: Antibodies like Ustekinumab target Interleukin-12/23.
- Crohn’s Disease and Ulcerative Colitis: TNF-alpha inhibitors are also used.
- Multiple Sclerosis: Some antibodies work to modulate the immune response to reduce disease attacks.
Benefits of Monoclonal Antibody Therapy
Monoclonal antibody therapy offers a set of tangible benefits that distinguish it from many traditional treatments, making it a preferred option in many cases.
First, high precision and targeting: This is the most prominent advantage, as monoclonal antibodies can bind to specific molecular targets on diseased cells without significantly affecting healthy cells.
This translates to fewer and more tolerable side effects compared to broad chemotherapy, which can affect rapidly dividing cells throughout the body. While side effects may occur with monoclonal antibody therapy, they are often less severe and more localized.
Second, the ability to achieve strong and long-lasting therapeutic responses: In many types of cancer and autoimmune diseases, monoclonal antibodies have led to sustained disease control, and some contribute to achieving cure in certain cases.
Third, improving patients’ quality of life: Thanks to reduced side effects and increased treatment effectiveness, patients can better continue their daily activities, which positively reflects on their overall quality of life.
Fourth, “personalized medicine“: Monoclonal antibodies allow for the design of treatment plans that match the unique molecular fingerprint of each patient’s disease, increasing the chances of treatment success and reducing ineffective treatments. These combined benefits make monoclonal antibody therapy a key driver of progress in precision medicine.
Side Effects of Monoclonal Antibody Therapy
Despite the high precision of monoclonal antibody therapy, it is not without side effects, which can vary significantly depending on the type of antibody used, the target it aims for, and the patient’s overall health.
One common side effect is infusion reactions, which may occur during or shortly after drug administration.
These reactions can include allergy-like symptoms such as fever, chills, nausea, rash, or difficulty breathing.
These reactions are usually managed by administering anti-allergy medications before infusion and by slowing the infusion rate.
Additionally, other side effects can occur depending on the antibody’s mechanism of action:
- Skin-related side effects: Such as rash, acne, or dry skin, especially with antibodies that target epidermal growth factor receptors (EGFR).
- Gastrointestinal side effects: Such as diarrhea or constipation, or nausea.
- Immune system-related side effects: In the case of immune checkpoint inhibitors, activating the immune system may lead to it attacking healthy tissues, causing inflammation in various organs such as the colon (colitis), liver (hepatitis), or endocrine glands (thyroiditis).
- Increased risk of infection: Some antibodies, especially those that suppress the immune system (such as TNF-alpha inhibitors), can increase the risk of infection.
Final Word
Monoclonal antibody therapy has proven to be not just a drug, but a qualitative leap in the methodology of medical treatment.
Through its unique ability to target specific molecules underlying diseases, this approach has provided precise and effective therapeutic solutions for previously intractable conditions.
It has transformed complex biological concepts into tangible treatments that make a real difference in the lives of patients worldwide.
Although challenges still exist, especially regarding cost and side effect management, continuous research and development in this field, supported by leading medical institutions such as Liva Hospital in Turkey, promise a future where treatments are more personalized, and safer.
Frequently Asked Questions
Is monoclonal antibody therapy the same as chemotherapy or immunotherapy?
Not exactly. Monoclonal antibody therapy is a type of targeted therapy (smart therapy), and it can also act as a type of immunotherapy if it targets immune checkpoints (like PD-1 inhibitors). However, it differs from chemotherapy, which broadly targets rapidly dividing cells.
What types of diseases does monoclonal antibody therapy treat?
It treats a wide range of diseases, most notably many types of cancer (such as breast, lung, colon cancer, lymphoma, melanoma); autoimmune and inflammatory diseases (such as rheumatoid arthritis, psoriasis, Crohn’s disease, multiple sclerosis); and some infectious diseases (such as COVID-19).
Can monoclonal antibody therapy be used for all cancer patients?
No, it cannot be used for all cancer patients. Its use depends on the presence of a specific “molecular target” (protein or mutation) in the patient’s cancer cells, which the antibody can target. This is determined through genetic and molecular tests on a tumor sample.
What are the common side effects of monoclonal antibody therapy?
Side effects vary depending on the specific drug, but they may include infusion reactions (such as fever, chills, rash), fatigue, nausea, and diarrhea. In some cases, especially with checkpoint inhibitors, immune-related side effects affecting various organs may occur.
How long does monoclonal antibody therapy last?
The duration varies greatly depending on the type of disease, the type of drug, and the patient’s response. Treatment may be given for a few months, several years, or even lifelong in some chronic autoimmune diseases. It is determined by the treating physician based on the patient’s condition.
Is monoclonal antibody therapy expensive?
Yes, monoclonal antibody drugs are typically very expensive due to the complexity of the research, development, and biological production processes. This presents a global challenge in making them accessible to everyone.